Biotechnology Gallery
HEALTH AND MEDICINE
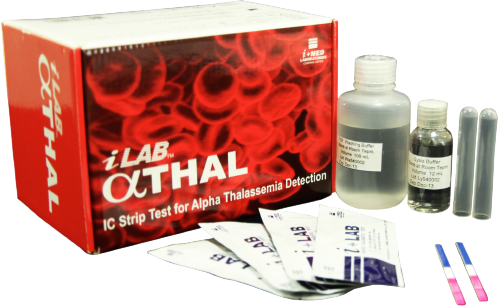
The alpha (α) thalassemia is an inherited blood disorder that causes anemia and other health problems. The disease is caused by mutations in genes that regulate the production of hemoglobin. PCR technique is commonly used to detect alpha thalassemia carrier but the technique has some limitations for the need of expensive equipment and well-trained technicians.
This new detection method is a lateral flow chromatographic immunoassay to detect Hb Bart’s in red blood cell hemolysates, which is usually present in a small amount in red blood cells of the carriers of alpha thalassemia 1. The technique is easy to perform, inexpensive, quick and no equipment necessary.
This technology has been licensed to i+MED Laboratories Company Limited and the product is available under the trade name “i+LAB αTHAL”.
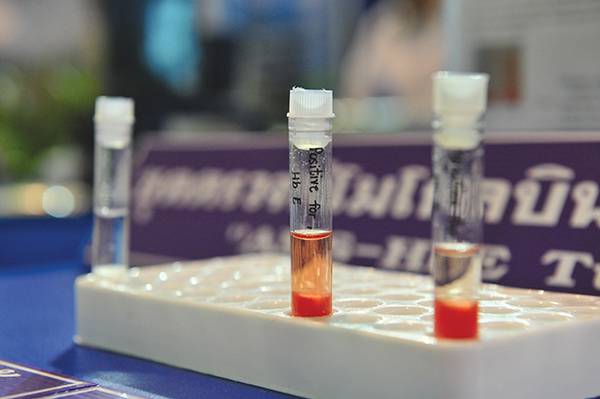
β-thalassemia is an inherited blood disorder which reduces the production of hemoglobin, the protein in red blood cells that carries oxygen to cell throughout the body. Hemoglobin E (HbE) is a b-structural variant of normal hemoglobin common worldwide. This HbE disorder can form a compound heterozygous state with the β-thalassemia gene, leading to life-threatening hereditary hemolytic anemia called HbE/ β-thalassemia. Screening of HbE has proven to be a challenging practice in prevention and control of the HbE/ β–thalassemia.
A convenient, ready- to-use screening test kit to screen HbE has been developed, using diethyl aminoethyl (DEAE)-cellulose resin. Accuracy and efficiency of the established method in detecting HbE is comparable with the standard cellulose acetate electrophoresis method. This method is inexpensive and simple with no requirement of sophisticated equipment. The method is highly suitable for mass population screening for HbE, particularly in small health care facilities.
This technology is under the licensing negotiation with a Thai company.

Dengue virus (DENV) causes a range of symptoms from mild dengue fever (DF) to severe dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS). Repeat infections with DENV are associated with severe DHF. Determination of dengue serotypes in current and previous epidemics will be crucial in being able to predict the severity of the current epidemics and for monitoring the predominant serotypes as a warning to a potential dengue outbreak.
The new assay “NS1-Serotyping-ELISA” was developed to detect the NS1 protein and identify DENV serotypes simultaneously. It offers an alternative to the current method of identifying DENV serotypes through laborious virus isolation followed by immunofluorescent assay and sophisticated RT-PCR.
This technology is available for licensing or further R&D collaboration. For further information, please contact NSTDA Technology Licensing Office (TLO).
Scientists constructed and tested the chimeric dengue viruses which comprise the prM-E coding region from recent dengue clinical isolates on the genetic background of attenuated virus. Based on promising results from the neurovirulence and immunogenicity testings in mice, the chimeric viruses are considered to have a good potential to be developed further as vaccine candidate for the safety and efficacy studies in non-human primate model. The four serotypes of dengue vaccine candidate were jointly developed by scientists from Chiang Mai University, Mahidol University and Medical Biotechnology Research Unit with research fund from NSTDA.
Four serotypes of chimeric live-attenuated vaccine candidate were licensed to BioNet-Asia Co., Ltd. in February 2011 to further develop into a commercial product.
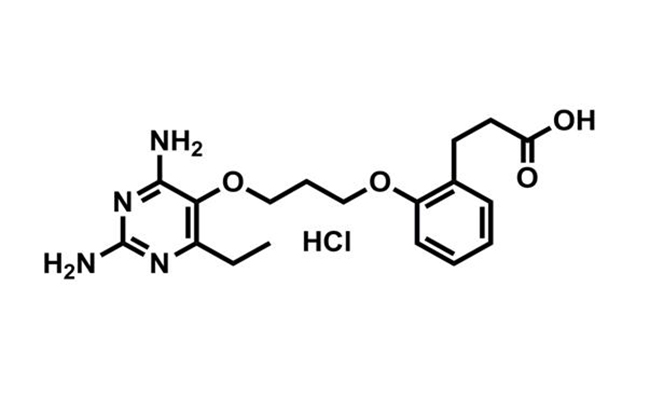
Malaria is an infectious disease caused by Plasmodiumspp. parasites. Current important problem of malaria treatment is the rise of parasites resistant to currently available antimalarial drugs. Thus there is an urgent need to develop new drugs that effectively kill drug resistant parasites.
BIOTEC research team, in collaboration with researchers from both local and international universities, has successfully developed new antimalarial drug candidate ‘P218’ that targets malarial enzyme dihydrofolate reductase (DHFR) and effectively kills both sensitive and resistant parasites.
P218 has been selected by Medicines for Malaria Ventures, a not-for-profit organization, for further development, and is currently undergoing a pre-clinical study.
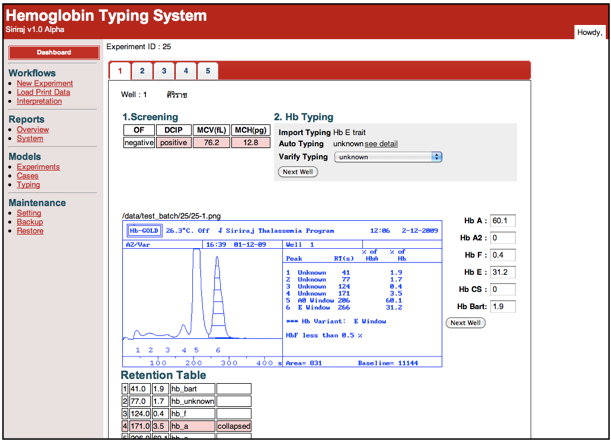
This tool was developed to interpret types of Thalassemia, Thalassemia carriers, and abnormal hemoglobin from hemoglobin type result obtained from Low Pressure Liquid Chromatography (LPLC). This tool, together with mean corpuscular volume (MCV) information, can be added-on to any LPLC for automatic thalassemia test result interpretation. This system uses computer and electronic technologies to intercept the print out signed from LPLC and analyze it together with MCV data to offer the thalassemia type interpretation for physicians and medical technicians.
This technology has been licensed to Drew-Bio (Thailand) Co., Ltd. for commercialization as an added-on feature to an LPLC.

TB-DNAsensor Kit is developed for the diagnosis of Mycobacterium tuberculosis (MTB), the causative agent of most cases of tuberculosis (TB). Based on the loop-mediated isothermalamplification (LAMP) technique, two types of test kit are available. TB-DNAsensor Kit I is a chromatographic on membrane for rapid, sensitive and specific MTB detection via amplification of target nucleotide sequences under inexpensive isothermal conditions with interpretation of results within 1.5 hour. TB-DNAsensor Kit II is a high sensitivity, specificity, rapid, low cost and convenient test for the detection of MTB from direct specimen such as sputum. The color change results can be detected from 45 minutes to 1.5 hour after reaction between gold nanoparticle and specific target DNA. Both Kits could be applied in laboratories and epidemiological surveys of MTB.
TB-DNAsensor Kit was developed by Srinakharinwirot University in collaboration with BIOTEC.
This technology is available for licensing. For further information, please contact Srinakharinwirot University.
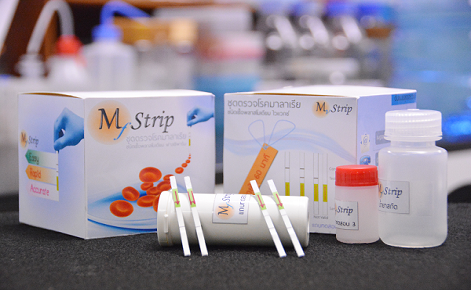
BIOTEC researchers have developed an assay platform using the loop-mediated isothermal amplification (LAMP) technique in combination with lateral flow dipstick for the detection of malaria parasite. The malarial dihydrofolate reductase–thymidylate synthase (dhfr-ts) gene, a target for antifolate class of anti-malarial drugs, is used as a target for this assay development. The total assay time of LAMP–LFD method is approximately 55 minutes, and the result can be visualized on the test strip. This novel method does not require sophisticated equipment, nor experienced technicians to interpret the result, making it highly suitable for field work and healthcare stations in remote area. The assays are available for Plasmodium falciparum and P. vivax.
This technology is available for licensing or further R&D collaboration. For further information, please contact NSTDA Technology Licensing Office (TLO).

Superdisintegrants are being used in the drug formulation to provide an immediate disintegration of tablets when being placed in a liquid environment leading to a rapid release of the active ingredient. BIOTEC researcher has developed the process to prepare cassava starch-based hydrogel which can be used as a superdisintegrant in drug tablets. Hydrogels are crosslinked polymeric materials which have an ability to absorb significant amounts of water in their three-dimensional polymeric matrix. The developed cassava starch hydrogel can quickly absorb water and swell very fast without the formation of viscous gel layer which may interfere with the release of the active material. This quick swelling of hydrogel particles exerts a tremendous force within the tablet and results in a rapid disintegration; thus, enhancing drug dissolution rate and improving its bioavailability. The developed hydrogel can also be applied in granules, capsules or other forms of solid preparation that require rapid release of the active compound.
This technology is ready to be transferred to entrepreneurs in the drug development industry.